|
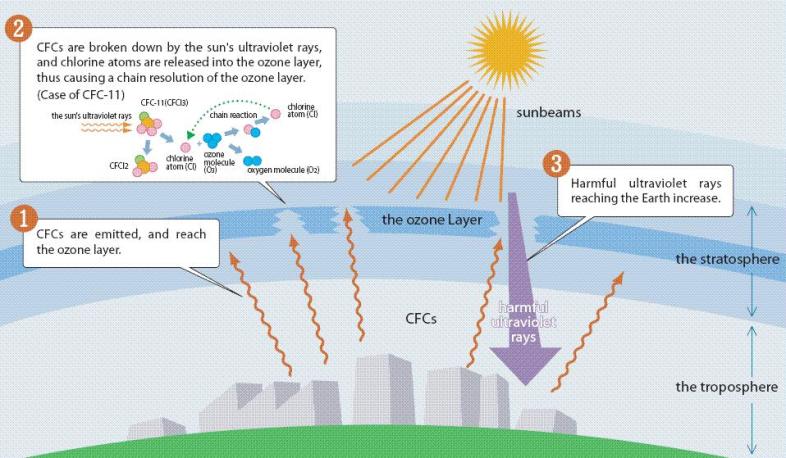
CFCs Chlorofluorocarbon (CFC) is an organic compound that contains carbon, chlorine, and fluorine, produced as a volatile derivative of methane and ethane. A common subclass is the hydrochlorofluorocarbons (HCFCs), which contain hydrogen, as well. Freon is DuPont's brand name for CFCs, HCFCs and related compounds. Other commercial names from around the world are Algofrene, Arcton, Asahiflon, Daiflon, Eskimo, FCC, Flon, Flugene, Forane, Fridohna, Frigen, Frigedohn, Genetron, Isceon, Isotron, Kaiser, Kaltron, Khladon, Ledon, Racon, and Ucon. The most common representative is dichlorodifluoromethane (R-12 or Freon-12). Chlorofluorocarbons (CFCs) are a family of chemical compounds developed back in the 1930's as safe, non-toxic, non-flammable alternative to dangerous substances like ammonia for purposes of refrigeration and spray can propellants. Their usage grew enormously over the years. One of the elements that make up CFCs is chlorine. Very little chlorine exists naturally in the atmosphere. But it turns out that CFCs are an excellent way of introducing chlorine into the ozone layer. The ultraviolet radiation at this altitude breaks down CFCs, freeing the chlorine. Under the proper conditions, this chlorine has the potential to destroy large amounts of ozone. This has indeed been observed, especially over Antarctica. As a consequence, levels of genetically harmful ultraviolet radiation have increased.
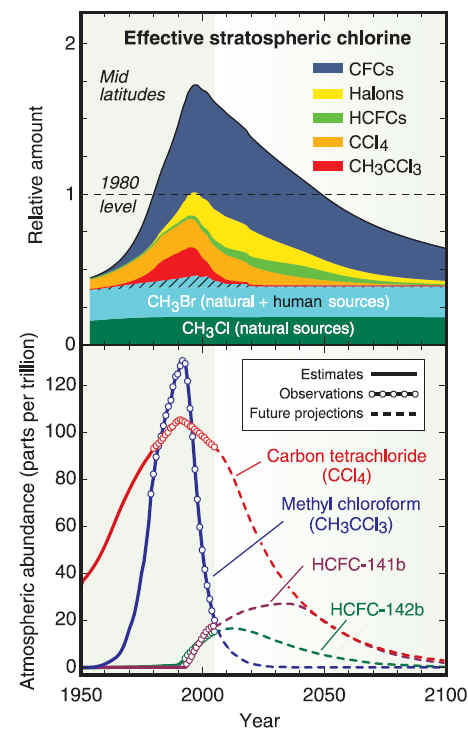
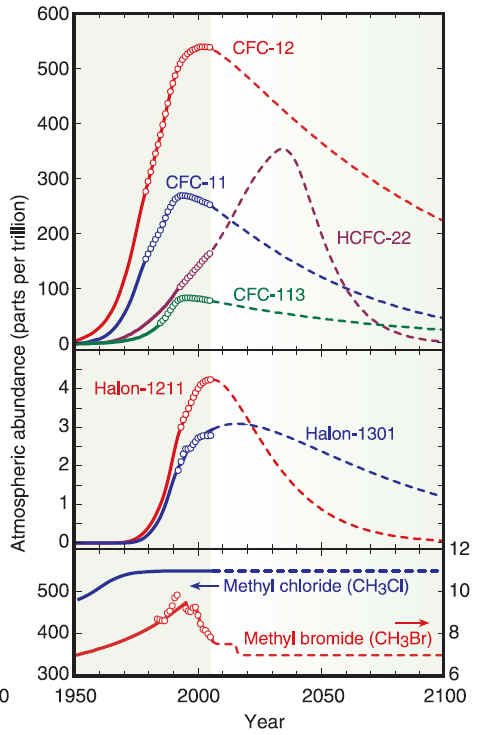
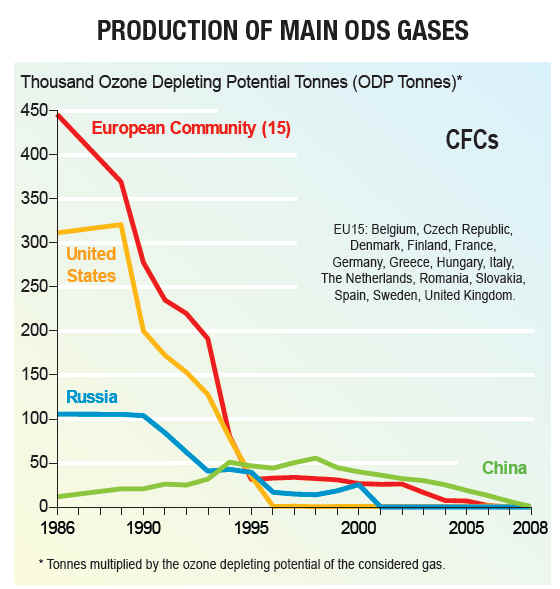
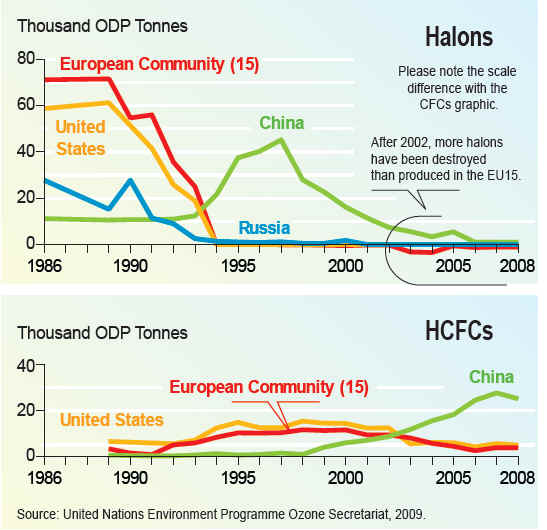
Production of new stocks ceased in most countries as of 1994 However many countries still require aircraft to be fitted with halon fire suppression systems because no safe and completely satisfactory alternative has been discovered for this application. There are also a few other, highly specialized uses. These programs recycle halon through "halon banks" coordinated by the Halon Recycling Corporation to ensure that discharge to the atmosphere occurs only in a genuine emergency and to conserve remaining stocks. Development of alternatives for CFCsWork on alternatives for chlorofluorocarbons in refrigerants began in the late 1970s after the first warnings of damage to stratospheric ozone were published. The hydrochlorofluorocarbons (HCFCs) are less stable in the lower atmosphere, enabling them to break down before reaching the ozone layer. Nevertheless, a significant fraction of the HCFCs do break down in the stratosphere and they have contributed to more chlorine buildup there than originally predicted. Later alternatives lacking the chlorine, the hydrofluorocarbons (HFCs) have an even shorter lifetimes in the lower atmosphere. One of these compounds, HFC-134a, is now used in place of CFC-12 in automobile air conditioners. Hydrocarbon refrigerants (a propane/isobutane blend) are also used extensively in mobile air conditioning systems in Australia, the USA and many other countries, as they have excellent thermodynamic properties and perform particularly well in high ambient temperatures. One of the natural refrigerants (along with Ammonia and Carbon Dioxide), hydrocarbons have negligible environmental impacts and are also used worldwide in domestic and commercial refrigeration applications, and are becoming available in new split system air conditioners
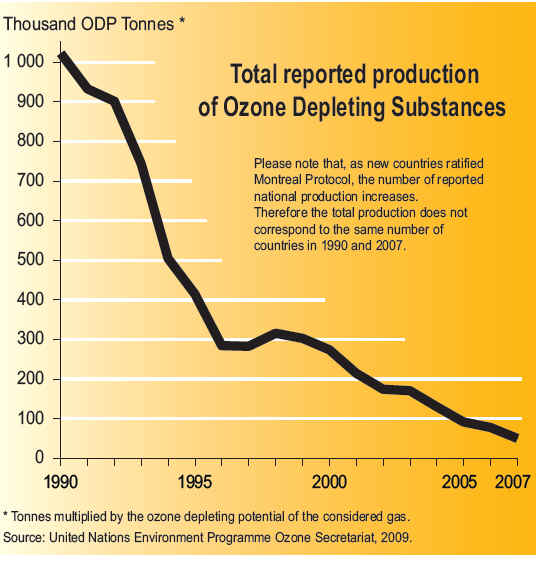
CFC History Refrigerators from the late 1800s until 1929 used the toxic gases, ammonia (NH3), methyl chloride (CH3Cl), and sulfur dioxide (SO2), as refrigerants. Several fatal accidents occurred in the 1920s because of methyl chloride leakage from refrigerators. People started leaving their refrigerators in their backyards. A collaborative effort began between three American corporations, Frigidaire, General Motors and DuPont to search for a less dangerous method of refrigeration. In 1928, Thomas Midgley, Jr. aided by Charles Franklin Kettering invented a "miracle compound" called Freon. Freon represents several different chlorofluorocarbons, or CFCs, which are used in commerce and industry. The CFCs are a group of aliphatic organic compounds containing the elements carbon and fluorine, and, in many cases, other halogens (especially chlorine) and hydrogen. Freons are colorless, odorless, nonflammable, noncorrosive gases or liquids. Chlorofluorocarbons (CFCs) are highly stable compounds that were used as propellents in spray cans and in refrigeration units. They are several organic compounds composed of carbon, fluorine, chlorine, and hydrogen. CFCs are manufactured under the trade name Freon . The invention of chlorofluorocarbons (CFCs) in the late 1920s and early 1930s stemmed from the call for safer alternatives to the sulfur dioxide and ammonia refrigerants used at the time, CFCs found wide application after World War II. Chloroflourocarbons were first created in 1928 as non-toxic, non-flamable refrigerants, and were first produced commercially in the 1930's by DuPont. The first Chlorofluorocarbon was CFC-12, a single carbon with two chlorines and two Fluorines attached to it. These halogenated hydrocarbons, notably trichlorofluoromethane (CFC-11, or F-11) and dichlorodifluoromethane (CFC-12, or F-12), have been used extensively as aerosol-spray propellants, refrigerants, solvents, and foam-blowing agents. They are well-suited for these and other applications because they are nontoxic and nonflammable and can be readily converted from a liquid to a gas and vice versa. Chlorofluorocarbons or CFCs (also known as Freon) are non-toxic, non-flammable and non-carcinogenic. They contain fluorine atoms, carbon atoms and chlorine atoms. The 5 main CFCs include CFC-11 (trichlorofluoromethane - CFCl3), CFC-12 (dichloro-difluoromethane - CF2Cl2), CFC-113 (trichloro-trifluoroethane - C2F3Cl3), CFC-114 (dichloro-tetrfluoroethane - C2F4Cl2), and CFC-115 (chloropentafluoroethane - C2F5Cl). CFCs have been found to pose a serious environmental threat. Studies undertaken by various scientists during the 1970s revealed that CFCs released into the atmosphere accumulate in the stratosphere, where they had a deleterious effect on the ozone layer. Stratospheric ozone shields living organisms on Earth from the harmful effects of the Sun's ultraviolet radiation; even a relatively small decrease in the stratospheric ozone concentration can result in an increased incidence of skin cancer in humans and in genetic damage in many organisms. In the stratosphere the CFC molecules break down by the action of solar ultraviolet radiation and release their constituent chlorine atoms. These then react with the ozone molecules, resulting in their removal. CFCs have a lifetime in the atmosphere of about 20 to 100 years, and consequently one free chlorine atom from a CFC molecule can do a lot of damage, destroying ozone molecules for a long time. Although emissions of CFCs around the developed world have largely ceased due to international control agreements, the damage to the stratospheric ozone layer will continue well into the 21st century. In 1978 The Montreal Protocol was adopted as a framework for international cooperation regarding CFC control on the basis of the Vienna Convention for the Protection of the Ozone Layer.
|
||||||||||||||||||