SOUTH POLE, ANTARCTICA– With the arrival of mid-August, comes our first glimpse of dawn on the horizon. Not only is this a welcome sight to us “Polies”, but it brings upon us initiation of the destruction of ozone in the stratosphere (location of the “ozone layer”). When I was in grade school I seem to remember that the ozone hole was all the rage as one of the primary environmental concerns. These days, it is far overshadowed by the rise of greenhouse gases and the study of climate change. The ozone hole has taken somewhat of a back seat in the public eye. And maybe that is a sign of success. In 1989, the Montreal Protocol was put into effect beginning the phase out of chlorofluorocarbons (CFCs). CFCs were used in things such as refrigerants, solvents, and aerosol sprays. We are now beginning to see a leveling off and even a decrease in some CFCs in the atmosphere. So is that an environmental problem that we chalk up as successfully solved? Well, maybe.
The atmosphere has four main layers which are the troposphere (the lowest layer in which most weather occurs), the stratosphere, mesosphere, and thermosphere. There is some ozone in the troposphere, but it is a very small amount and is produced by the reaction of pollution and ultraviolet (UV) light. Most of the ozone in the atmosphere is located in the stratosphere, hence the name the “ozone layer”. The ozone layer is important because it filters out some harmful UV radiation. CFCs eventually make it into the stratosphere and mix in with the ozone molecules. The CFCs don’t do their damage until they react with UV radiation which breaks the bond of the chlorine or bromine atom apart from the rest of the CFC molecule. Chlorine and bromine are highly reactive with ozone (a molecule consisting of 3 oxygen atoms) which then breaks the ozone apart becoming a ClO and O2 (regular breathable oxygen). The creation of ozone in the stratosphere is from the interaction of UV radiation with an O2 molecule. It splits the O2 creating two single oxygen atoms which then react with O2 creating O3 (ozone).
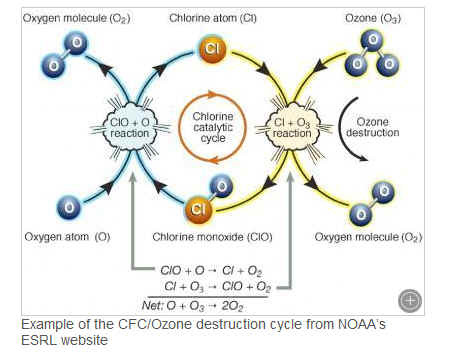
These appear to be processes that could take place all over the world so why is the ozone hole unique to the Antarctic region? There are two main factors that enable a hole in the ozone layer to form over Antarctica: the polar stratospheric vortex and polar stratospheric clouds. Antarctica’s extreme cold temperatures allow for these polar stratospheric clouds to form. The clouds enhance CFC/ozone reaction causing the destruction of ozone to become very effective. The polar stratospheric vortex forms every winter over the Antarctic continent and keeps the air from interacting outside of the vortex. So basically ozone from outside the vortex is unable to flow in and replenish during this time. That is when we see the lowest ozone values. As temperatures warm through the summer, the polar stratospheric vortex begins to break up, polar stratospheric clouds disappear, and the air mixes back into the Antarctic stratosphere replenishing the ozone layer. The filling in of the ozone hole causes a decrease in ozone worldwide which how it becomes a worldwide issue. The ozone hole hasn’t proved to be decreasing yet but the fact that the harmful CFCs look to be working their way out of the atmosphere is encouraging and we look for the hole to begin decreasing in decades to come.
So right now we are at the point where the sun is getting just high enough (still well below the horizon) that its rays are beginning to hit the stratosphere breaking down the CFCs that are up there. At the Atmospheric Research Observatory (ARO) we measure ozone in three different ways. One is with a surface analyzer that gives us a baseline level of tropospheric ozone, and the other two include the Dobson Spectrophotometer and launching ozonesondes which both give us an idea of stratospheric ozone.
When there is enough light outside for video, I will take you through an entire launch sequence explaining how we prep the sonde, prep the balloon, launch the balloon and show the software that gives us the profile of the data. We can even compare what a normal “healthy” layer looks like prior to the hole forming to the hole itself.








