|
TWENTY YEAR ANNIVERSARY OF
THE DISCOVERY OF THE CAUSE OF THE ANTARCTIC OZONE HOLE — A SCIENTIFIC
SUCCESS STORY

Susan Solomon
credit:NOAA
September 15, 2006 NOAA Magazine — Twenty years ago this August, NOAA senior scientist Susan Solomon, along with other government and university scientists, identified the cause of the Antarctic ozone hole — a discovery that is still being celebrated as the most significant scientific/global environmental success story of the 20th century. Within five years of the hole’s discovery by the British Antarctic Survey, Solomon’s proposed chemistry for the linkage of increases in long-lived man-made chlorofluorocarbons (or CFCs) primarily responsible for the seasonal Antarctic ozone hole was confirmed and the Montreal Protocol on Substances that Deplete the Ozone Layer (the first treaty to address the Earth's environment) was enacted to phase out these compounds. As a result, the global production of these ozone-depleting compounds is now greatly reduced and there are signs that the ozone hole is slowly stepping into a recovery phase — both CFC and ozone levels are showing signs of leveling off and some CFCs have even started to decrease. "This is an example of the quality science conducted by NOAA scientists and their colleagues, often in extreme conditions, and how it informs those who make decisions that affect our daily lives," said retired Navy Vice Adm. Conrad C. Lautenbacher, Ph.D., undersecretary of commerce for oceans and atmosphere and NOAA administrator. "Through this work, the nation has gained a better understanding of our atmosphere, which is a key element of NOAA's mission." David Hofmann, director of the NOAA Global Atmospheric Monitoring Program described the status of the ozone layer today as, "the patient hasn't recovered, but it's not getting any sicker. We really have not seen any recovery in Antarctica." NOAA predicts that it could take until 2060 for the ozone layer to heal completely, provided humans stop all release of man-made substances containing chlorine (or bromine). The Ozone Layer The ozone layer is a thin, invisible layer of the Earth's atmosphere about 15 miles thick. In nature, ozone production and destruction are balanced, but the introduction of man-made compounds has upset this balance. Much like sunscreen for the Earth, the ozone layer shields the Earth from the sun’s damaging UV-B radiation, which can adversely affect human health and ecosystems. The Ozone
Depletion Story The History and
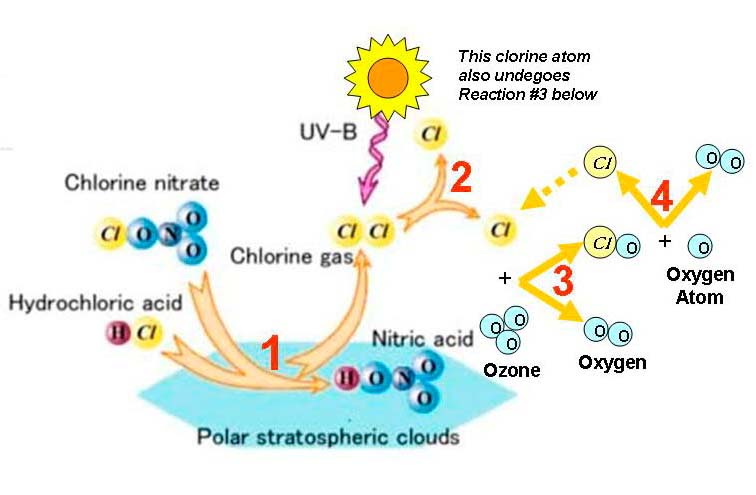
the Science behind the Antarctic Ozone Hole In fact, by 1983, a United States National Research Council report projected that continued use of CFCs at then-current rates would probably lead to depletion of the total global ozone layer by only about three percent in about a century. Many argued that this was a small effect, far in the future, and subject to large uncertainties. Unexpected Discovery of the Antarctic Ozone Hole The situation changed dramatically with the publication of the discovery of the Antarctic ozone hole by British Antarctic survey scientist, Joseph Farman, and his colleagues in 1985. Although the British researchers first doubted the validity of their own measurements — taken in the springtime ozone layer above Halley Bay, Antarctica — their work was quickly confirmed by measurements from satellites and from other Antarctic research stations. Having monitored ozone levels at that site for decades, Farman noticed that the ozone layer thinned every spring (starting in August/September and reaching a minimum in early October) over Antarctica and that the thinning started to increase in the late 1970’s. Ozone appeared to be depleted not by a few percent, but by about a third and not in the far future, but just a few years after the National Research Council said little would occur for a century. This and the fact that observed ozone losses occurred concurrently with increases of CFC-11 and CFC-12 concentrations in the troposphere raised fears that ozone depletion may have been drastically underestimated. Furthermore, the ozone depletion was not occurring at the very top of the ozone layer (near 40 kilometers), as expected from gas-phase chlorine chemistry, but at an entirely different height range from about 10-20 kilometers (the very heart of the ozone layer). It was clear that this ozone depletion was not only larger than had been expected, but totally different in character. It could not be explained by the then-current ozone depletion theories involving “gas phase” chemistry, so a massive shift in scientific understanding was needed to explain this change in ozone depletion from global to polar, and from 40 kilometers down to 10-20 kilometers. Antarctic Ozone
Hole Ozone Theories
Solomon’s Theory Subsequent laboratory and field measurements (from the ground, balloon borne and from high-altitude airplanes) confirmed Solomon’s theory that the ozone hole was caused by the interaction of CFCs, sunlight and the icy clouds that form in that special region of the Earth. Scientists had gravely underestimated the chemicals' destructive power, and the ozone layer faced even more danger than previously thought. Observational
Evidence: Verifying the Role of CFCs and Surface Chemistry Solomon was an atmospheric chemist at the then-NOAA Aeronomy Laboratory (now the NOAA Chemical Sciences Division) in Boulder, Colo., when she led the NOAA team. David Hofmann, then with the University of Wyoming, headed the UW team. He later joined NOAA and is now the Director of ESRL’s Global Monitoring Division. Both were funded by NSF, which operates the McMurdo Station at the South Pole. Researchers from NASA and the State University of New York (SUNY) at Stoneybrook, rounded out the scientific party. Other sponsors included NOAA, the Chemical Manufacturers Association, the U.S. Navy and ITT Antarctic Services.
NOAA Measurements
at McMurdo Station The falling ozone was accompanied by a spectacular enhancement in chlorine dioxide. Chlorine dioxide was a particularly important early measurement because that molecule is proportional to other reactive forms of chlorine (i.e., Cl and ClO). Solomon’s measurements of greatly enhanced chlorine dioxide therefore showed that the polar stratospheric clouds were indeed liberating chlorine. The nitrogen dioxide was in contrast extremely low, further confirming expectations based upon polar stratospheric cloud chemistry. The proposed surface chemistry converts nitrogen-containing gases to nitric acid (HNO3), which impedes the reformation of “unreactive” chlorine nitrate (ClONO2) and thereby further enhances the ozone loss by allowing chlorine to stay in its active ozone-destroying forms longer. As temperatures rose in early October, the chlorine dioxide disappeared and the nitrogen dioxide increased, again as expected. Thus the seasonal behavior of both molecules supported Solomon’s theory. Furthermore, as verified by the University of Wyoming balloon measurements, the ozone losses were confined primarily to the lower stratosphere, between about 12 and 20 kilometers, as would be expected for the new surface ozone depletion chemistry. In the end, all four of the major NOZE teams successfully measured the key chlorine and nitrogen containing compounds indicative of the new surface ozone depletion chemistry. This data, along with additional findings from the NOZE II mission in 1987 and the Airborne Antarctic Ozone Experiment (also known as AAOE) the same year, showed conclusively that human-produced trace gases that contain chlorine (and bromine) had caused the ozone hole. Montreal Protocol on Substances that Deplete the Ozone On Sept. 16, 1987, diplomats from around the world met in Montreal and forged a treaty unprecedented in the history of international negotiations. Environmental ministers from 24 nations, representing most of the industrialized world, agreed to set sharp limits on the use of CFCs (and Halons). More Ozone Depletion in the North Having seen that chlorine dioxide was greatly enhanced in Antarctica, Solomon and her colleagues went north to Thule, Greenland in Jan. 1988, to search for enhanced chlorine chemistry in the Arctic. Indeed there were clear signs that chlorine dioxide was also significantly enhanced in the north compared to gas-phase chemistry, but at levels much lower than those observed in the Antarctic (as would be expected since Arctic temperatures usually warm up in spring much earlier than in the Antarctic, limiting the overlap between sunlight and cold temperatures and thus ozone depletion). Therefore, both the Antarctica and Arctic polar ozone loss appeared to depend upon the overlap between chemical perturbations due to cold temperatures, associated polar stratospheric clouds and sunlight. 1990 London Amendments The fast-paced research of the late 1980s led diplomats to conclude that the original Montreal Protocol would not go far enough toward protecting the fragile ozone layer. Therefore, in June 1990, diplomats met in London and voted to significantly strengthen the Montreal Protocol. New Phases in
Understanding Surface Chemistry Furthermore, NOAA scientists Hofmann and Solomon realized that the abundances of ozone depleting substances were greatly enhanced after explosive volcanic eruptions. Solomon and her colleagues have made several contributions to the understanding of liquid phase chemistry in the stratosphere, particularly regarding the role of volcanic effects. While the discovery and explanation of the Antarctic ozone hole was important to policy deliberations, the identification and explanation of ozone loss at mid-latitudes helped inspire further action by policymakers to reduce emissions of CFCs (e.g., Copenhagen and Beijing Amendments). Closing thoughts
Solomon and her NOAA colleagues have been involved in multiple phases of understanding of ozone loss chemistry both through modeling and observations. The picture has evolved from the gas-phase alone to solids and liquids. Further, the understanding of liquid phase chemistry has led to an evolution of thinking from extreme cold to less extreme, expanding both the processes that must be considered and where they can occur in altitude and latitude.
Ozone Depletion in the Antarctic Springtime
|